- Visibility 531 Views
- Downloads 177 Downloads
- Permissions
- DOI 10.18231/j.ctppc.2024.034
-
CrossMark
- Citation
A concise review- Development of an analytical method and validation of dutasteride
- Author Details:
-
Pritam S Jain *
-
Hina Chaudhari
-
Mayuri Chaudhari
-
NG Haswani
Abstract
The synthetic 4-azasteroid dutasteride inhibits the type 1 and type 2 isoenzymes of 5-alpha-reductase in a selective and competitive manner. Men with symptomatic benign prostatic hyperplasia (BPH), which is characterized by an enlarged prostate, may use it. Orally administered dutasteride has a 60% bioavailability and is quickly absorbed.
This work focuses on the most recent developments in analytical techniques for quantifying dutasteride in various biological samples (including human plasma), as well as in commercially available dose forms and bulk. High-performance thin-layer chromatography (HPTLC), gas chromatography in conjunction with tandem mass spectrometry (GC-MS), high-performance liquid chromatography (HPLC), and UV spectroscopy will all be thoroughly examined in this review. The matrix, stationary phase, mobile phase composition, detection wavelength, RF value, retention duration, detection limit (DL), carrier gas, and flow rate are just a few of the characteristics that will be covered.
Introduction
Type 1 and type 2 variants of the 5α-reductase enzyme, which converts testosterone into 5α-dihydrotestosterone (DHT), are specifically inhibited by dutasteride. Given that DHT is crucial in prostate enlargement, dutasteride is employed to treat benign prostatic hyperplasia, a condition frequently affecting men over the age of 50. [1]
5-α, 17β)-N-{2,5-bis (trifluoromethyl)phenyl}-3-oxo-4-azaandrost-1-ene-17-carboxamide is the chemical name for dutasteride (DTS). C27H30F6N2O2 is its empirical formula, and its molecular weight is 528.5 grams per mole. [2]
Dutasteride is an intracellular enzyme that transforms testosterone; it is used to treat patients with benign prostatic hyperplasia who exhibit symptoms.Dutasteride belongs to the class of 5α-reductase inhibitors, which selectively and competitively block both type 1 isoforms (active in sebaceous glands across various skin areas and the liver) and type 2 isoforms (primarily located in reproductive tissues like the prostate, hair follicles ,seminal vesicles, epididymides and liver). [3], [4]
A review of the literature shows that numerous analytical and bioanalytical techniques have been developed for analyzing dutasteride. These techniques, which can be applied alone or alongside other medications, include high-performance liquid chromatography (HPLC). [5], [6], [7]Dutasteride dissolves well in organic solvents such as methanol, dimethyl sulfoxide, ethanol, acetone, and isopropanol but is nearly insoluble in water. The USP Medicines Compendium lists it as a medication only meant for monograph development. It works by preventing testosterone from being converted to 5α-dihydrotestosterone.
After being approved by the USFDA in October 2002, it has subsequently been granted authorization in a number of other nations. DHT is the primary androgen responsible for the initial growth and subsequent enlargement of the prostate gland. [8], [9]
Structure
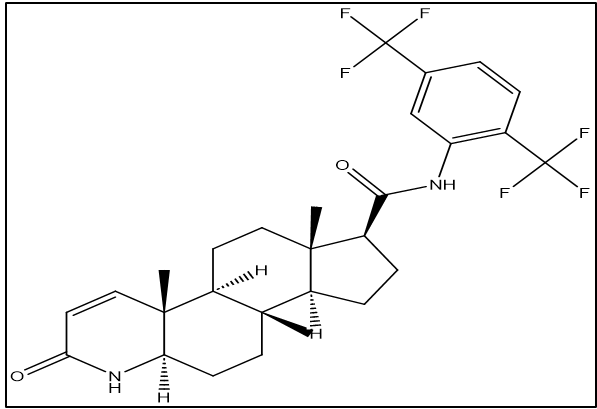
Pharmacodynamic Profile
Dutasteride's effect on 5α-reductase inhibition has been investigated in lab settings. However, research on how dutasteride affects levels of dihydrotestosterone (DHT), testosterone, luteinizing hormone (LH), and prostate-specific antigen (PSA) has predominantly been conducted with volunteers or patients suffering from benign prostatic hyperplasia (BPH)[10], [11] The effects of dutasteride on spermatogenesis, lipid levels, and bone metabolism have also been examined in volunteer studies.[12]
Activity that inhibits 5α-reductase
The 4-azasteroid dutasteride is a very strong and selective inhibitor of type 1 and type 2 5α-reductase.Top of FormBottom of Form In laboratory studies, its inhibition constants (Ki) are 6 nmol/L for type 1 5α-reductase and 7 nmol/L for type 2 5α-reductase. [10], [11] Dutasteride operates about five times faster and is 45 times more potent in inhibiting type 1 5α-reductase than finasteride, which has a Ki of 360 nmol/L. Additionally, for inhibiting type 2 5α-reductase, dutasteride is estimated to be 2.5 times more potent than finasteride. [11], [13], [14]
Impact on DHT and other hormone levels
The impact of dutasteride on serum hormone levels has been studied through three randomized, placebo-controlled, dose-ranging trials involving male volunteers or patients with benign prostatic hyperplasia (BPH). Dutasteride administration led to increases in luteinising hormone and androstenedione, but these changes were considered not clinically significant. [15], [16], [17] In the single-blind study with 48 volunteers, participants were administered a single dose of either dutasteride (between 0.01 and 40 mg), finasteride 5 mg, or a placebo. In contrast, the double-blind studies, which included patients with a prostate volume of at least 30 mL or an International Prostate Symptom Score (IPSS) of 8 or higher, had durations of 4 weeks (with 53 participants) or 24 weeks (with 399 participants) [17]
Dutasteride caused dose-dependent decreases in average DHT levels in both volunteers and patients who took the drug daily for 4 or 24 weeks. Reductions in DHT levels generally stabilized at doses above 0.5 mg, which is the recommended dosage. After a single dose of dutasteride ranging from 1 to 40 mg, DHT levels in volunteers were significantly reduced by 72–95% compared to baseline (all p ≤ 0.001 versus placebo For patients who received daily doses of dutasteride ranging from 0.05 to 5 mg for 24 weeks, DHT levels were reduced by 52.9–98.4% from baseline (all p < 0.001 versus placebo) [16], [17]
Impact on PSA Levels
PSA levels declined after treatment with dutasteride. [18], [19] In an individual analysis of one of the ARIA trials (n = 674), the mean baseline levels of total and free PSA in patients receiving dutasteride were 4.4 and 0.9 ng/mL, respectively. After 12 months of treatment, these levels were reduced by an average of 45.7% and 55.5%. [19] In contrast, with placebo, total PSA levels increased by an average of 8.4%, rising from 4.1 ng/mL at baseline, and free PSA levels increased by an average of 14.0%, rising from 0.9 ng/mL at baseline. The free-to-total PSA ratio followed a similar pattern, decreasing by an average of 6.7% in patients receiving dutasteride and increasing by an average of 6.7% in those receiving placebo. [19]
Pharmacokinetic Profile
As of February 2006, there were no published pharmacokinetic studies specifically focused on dutasteride; all the data reviewed were obtained from the manufacturer's prescribing information. Generally, the pharmacokinetic data available were mostly from studies involving healthy volunteers. [20]
Absorption
Dutasteride is rapidly absorbed and shows dose-proportional increases in mean maximum plasma concentrations (Cmax) after oral administration. Its bioavailability is approximately 60% when taken orally.[21] Following the administration of dutasteride in doses ranging from 0.1 to 40 mg, the absorption lag time (tlag) was 0.32 hours, as determined by the two-compartment model.[10]
Distribution
Food intake decreases the maximum serum concentrations of dutasteride, though this reduction is not deemed clinically significant. Once in the bloodstream, dutasteride is extensively bound to albumin (99%) and alpha-1 acid glycoprotein (96.6%) and is broadly distributed throughout both central and peripheral compartments and has a substantial volume of distribution, ranging between 300 and 500 liters.The drug also distributes to semen, where its concentration is about 10% of the serum concentration.[22]
Metabolism
Dutasteride is extensively metabolized, mainly by the CYP3A4 and CYP3A5 enzymes.[22] Dutasteride undergoes extensive metabolism and is mainly excreted in the feces. At steady state, approximately 5.4% (with a range of 1.0% to 15.4%) of the 0.5 mg/day dose of dutasteride is excreted in the feces as unchanged drug.[23]
Elimination
The elimination of dutasteride is dose-dependent and is best characterized by a combination of linear and nonlinear pathways functioning concurrently. At high plasma concentrations of dutasteride (daily doses greater than 1 mg), the linear pathway becomes the primary route of elimination, leading to a clearance rate of 0.58 L/h. [10]
Analytical accounts on Dutasteride
The thorough literature analysis identified a number of analytical techniques, such as UV spectrophotometry, HPLC, HPTLC, and LC-MS/MS, for detecting dutasteride in both bulk materials and pharmaceutical formulations. These techniques describe how to analyze dutasteride in matrices like human plasma and in various dose forms, including tablets.
Spectrophotometric Overview
UV-Visible spectroscopy method
Mohammad Kaisarul Islam et. al. outlined a simple visible spectrophotometric technique for measuring Dutasteride in both raw materials and pharmaceutical formulations.Top of FormBottom of FormThe linearity of the method was assessed across seven concentration levels, and in the concentration range of 12 µg/ml to 28 µg/ml, Beer's law was followed. It was discovered that the correlation coefficient (r) for the standard curve was 0.997. In pharmaceutical formulations, the percentage recovery of dutasteride varies between 100% and 101%.employing a spectrophotometer with a blank of a 50:50 methanol:water mixture and a wavelength range of 200–380 nm. Dutasteride's absorption curve showed a clear absorption maximum at 241 nm.[24]
Mahtab Ali et. al. presented a simple visible spectrophotometric method for quantifying dutasteride in both bulk materials and pharmaceutical products. A linear relationship between absorbance and concentration was demonstrated by the calibration curves, and Beer's law was followed over a concentration range of 5-100 µg/mL. The correlation coefficient (r) for the standard curve was 0.9998. The recovery percentages for the dutasteride stock solution and the dosage form solution were 99.79% and 100.00%, respectively.[25]
Vishnu P. Choudhari et. al.described a straightforward visible spectrophotometric technique for analyzing dutasteride in both bulk quantities and pharmaceutical dosage forms. The first technique involves measuring derivative amplitudes at certain wavelengths and is referred to as the first-order derivative spectroscopy method (Method A). Area Under the Curve Spectrophotometry (Method B) is the second technique. In the first-order derivative spectra, amplitudes at 247.67 nm were chosen for determining DUTA, while the wavelength range of 237.13-238.25 nm was selected for DUTA determination using the AUC method in methanol. Beer's law is applicable to DUT A in Method A within a concentration range of 10–50 µg/mL, while it is applicable to DUTA in Method B within a range of 5–25 µg/mL. The recovery rate for DUTA using the first-order derivative spectroscopic method ranged from 99.60% to 99.99%. The recovery for DUTA using the AUC method ranged from 99.30% to 101.12%.[26]
AVVNKS Kumar et. al. developed four straightforward and sensitive visible spectrophotometric methods for the assay of dutasteride. Method I is based on the interaction of an acetonitrile solution with dutasteride, an electron donor, and chloranil, a π-acceptor. A reddish-orange chloranil radical anion is produced by this reaction, and its peak absorption occurs at 525 nm.In Method II, ferric chloride and an acidic environment are used to drive an oxidative coupling reaction between dutasteride and 3-methyl-2-benzothiazolinone hydrazone hydrochloride. The result of this reaction has a green hue with a peak absorption at 550 nm.The creation of ion couples between dutasteride and the dyes bromothymol blue and bromophenol blue is required for Methods III and IV. After the ion pairs are extracted into chloroform, absorption maxima for bromothymol blue and bromophenol blue are found at 425 and 435 nm, respectively. Regression analysis of the Beer’s law plots showed a strong correlation within the concentration ranges of 2-40 µg/mL for Method I, 1-20 µg/mL for Method II, 5-50 µg/mL for Method III, and 2-20 µg/mL for Method IV.[27]
Kareem, Z. M et. al. introduced a novel spectrophotometric flow injection method for measuring Dutasteride (DS) in both its pure form and in pharmaceutical tablet formulations. This procedure is predicated on the way that dutasteride, an electron donor, and chloranil, an acceptor, interact with acetonitrile. A reddish-orange Chloranil radical anion is produced by this reaction, and its peak absorption occurs at 525 nm. The technique exhibits linearity with a correlation coefficient of 0.9969 over a concentration range of 0.9948 to 8.932 µg/mL. It has a relative standard deviation (RSD) of 0.422% and a detection limit of 0.420 µg/mL.[28]
|
Sr. No. |
Drug |
Matrix/ Dosage form |
Solvent |
Detection (nm) |
Linearity (ug/ml) |
Ref. No. |
|
|
1 |
Dutasteride |
Tablet |
methanol-water (50:50) |
241 nm |
12-28 µg/ml |
24 |
|
|
2 |
Dutasteride |
Tablet |
Water,Ethanol, Methanol,Polyet Hylene glycol |
242 nm |
5-100 µg/mL |
25 |
|
|
3 |
Dutasteride |
Tablet /Capsule |
methanol |
(Method A) -247.67 nm |
10-50 µg/mL |
26 |
|
|
(Method B) -237.13 nm |
5-25 µg/mL |
||||||
|
4 |
Dutasteride |
Tablet |
Method-I |
500 mg Chloranil in 100ml of acetonitrile |
525 nm |
2-40 µg/mL |
27 |
|
Method-II |
3-methyl-2-benzothiazolinone hydrazone hydrochloride in the presence of ferric chloride |
550 nm |
1-20 µg/mL |
||||
|
Method-III |
bromothymol blue and bromophenol blue |
425 nm |
5-50 µg/mL |
||||
|
Method- IV |
435 nm |
2-20 µg/mL |
|||||
|
5 |
Dutasteride |
Tablet |
500 mg Chloranil and 100 ml acetonitrile |
525 nm |
0.9948-8.932 µg/mL |
28 |
Chromatographic overview
HPLC method
Taraka Ramesh G et. al. outlined a stability showing RP HPLC method for the estimation of dutasteride in tablet dosage formRP-HPLC separation was carried out in isocratic mode using a Symmetry C18 column (4.6 x 250 mm, 5μm, XTerra brand). The mobile phase was composed of 80% HPLC-grade acetonitrile and 40% phosphate buffer with the pH adjusted to 3.0 using orthophosphoric acid. A DAD detector was used for detection at 280 nm, and the flow rate was kept constant at 1.0 mL per minute. The entire run took place in 8 minutes. Dutasteride had a retention time of approximately 2.2 minutes.[29]
Abhilasha V.Deshmukh et. al. outlined a stability showing RP HPLC method for the estimation of armodafinil in tablet dosage form. Using a BDS HYPERSIL C18 (4.6 mm × 250 mm) analytical column, the method's development aimed to provide an accurate, dependable, and robust RP-HPLC approach for the quantitative determination of Dutasteride (DTS) in bulk materials and single-component formulations. The mobile phase included a 75:10:15 (V/V/V) ratio of acetonitrile, water, and methanol, a flow rate of 0.7 mL/min, and UV detection at 274 nm. Dutasteride was effectively resolved and eluted at 8.34 minutes, with a total run time of 10 minutes and the column temperature maintained at 20°C.[30]
G. Sravan Kumar Reddy et. al.described a stability-indicating RP-HPLC method for quantifying dutasteride in tablet dosage forms. A Symmetry C18 column (4.6 x 150 mm, 5 µm, XTerra brand) was used in isocratic mode for the RP-HPLC separation. 20% phosphate buffer, pH-adjusted to 2.5 with orthophosphoric acid, and 80% acetonitrile (HPLC grade) made up the mobile phase. Detection was done at 274 nm while the flow rate was kept at 0.8 mL per minute.Dutasteride exhibited a retention time of 2.003 minutes.[31]
Dr. Jadhav S. B et. al. developed a stability-indicating RP-HPLC method for the quantification of dutasteride in tablet dosage forms. A Phenomenex C18 column (250 mm x 4.6 mm, 5 μm particle size) was used as the stationary phase for the separation. The mobile phase was an 80:20 v/v mixture of methanol and water that was utilized in an isocratic mode at a flow rate of 1.0 mL/min. The retention period for dutasteride was 2.94 minutes. For drug concentrations between 1 and 5 μg/mL, the procedure showed linearity (r2 = 0.9999). In the investigation on forced degradation, dutasteride degraded 16.82% after three hours in 0.1 N HCl. The alkaline degradation study resulted in an 11.85% degradation, while the overall observed degradation for dutasteride was 4.10%.[32]
Ch Maratha Martini et. al. developed a stability-indicating RP-HPLC method for estimating dutasteride in tablet dosage forms. A Kromasil C18 column (150 mm × 4.6 mm, 5 µm particle size) was used for the isocratic method of chromatographic separation, and the mobile phase consisted of phosphate buffer and acetonitrile mixed 30:70 (v/v). Dutasteride showed a retention time of 7.2 minutes when the eluents were detected at 242 nm. A flow rate of 0.8 mL/min was selected. The method showed linearity for dutasteride concentrations ranging from 0.25 to 1.5 µg/mL, with a correlation coefficient of 0.999.
Dr. Keerthana Diyya et. al. developed a stability-indicating RP-HPLC method for estimating dutasteride in tablet dosage forms. An XTerra C18 column (150 × 4.6 mm, 5 µm particle size) was used as the stationary phase to produce the separation. Ammonium dihydrogen phosphate buffer (pH 6.5) and methanol were combined in an isocratic mobile phase at a flow rate of 1.0 mL/min in a 25:75 ratio. A UV detector was used to carry out the detection at 246 nm. The dutasteride retention time under these ideal circumstances was 4.26 minutes. Regression analysis revealed a good correlation (R² = 0.999) between 1.25 and 7.5 µg/mL for dutasteride, indicating that the approach was linear. The percentage recovery of dutasteride from the pharmaceutical dosage form was found to be 99.23%.[33]
Syed Hafeez Ahmed et. al.developed a stability-indicating RP-HPLC method for quantifying dutasteride in tablet dosage formsAn Inertsil ODS 3V C18 column (150 x 4.6 mm, 5 µm) was used for the chromatographic separation. Thirty liters each of acetonitrile, methanol, and 20 mM ammonium acetate buffer (pH 3.5) made up the mobile phase. Detection was performed at 223 nm. The method showed linearity for dutasteride concentrations ranging from 24 to 56 µg/mL, with a correlation coefficient (r²) of 0.998. [34]
Patel Virendra Kumar et. al. describe RP-HPLC as an innovative analytical method suitable for detecting dutasteride in both pharmaceutical formulations and bulk drug substances. The goal in developing this technique was to establish a reliable and precise RP-HPLC method using a Shiseido C18 analytical column for accurately measuring the concentration of dutasteride in both bulk and single-component forms. MeOH, ACN, and H2O were combined in a 75:10:15 (V/V/V) ratio to form the mobile phase. A constant flow rate of 0.7 mL/min was employed for detection, and the UV wavelength was set at 274 nm. Dutasteride had a retention time of 8.34 minutes. The calibration plot displayed a linear correlation within the concentration range of 10 to 22 ppm.
Vaishali A. Shirsat et. al. developed a novel, simple, and sensitive reverse phase high-performance liquid chromatography (RP-HPLC) method for performing forced degradation studies on dutasteride. On a Hi-Q Sil C18HS column, DTS and its degradants were separated using a linear gradient elution, and UV detector detection was employed. The mobile phase for the isocratic mode of DTS separation from its degradants was a 20:80 (v/v) mixture of methanol and 10 mM ammonium acetate buffer (pH 4.5).The mobile phase in the linear gradient mode was composed of methanol and 10 mM ammonium acetate (pH 4.5), with the following composition changes: For the first two minutes, split the ratio 50:50; after two minutes, switch to 20:80; after twenty minutes, go back to 50:50; and after thirty minutes, stay at 50:50. A UV wavelength of 225 nm was used for detection, and the flow rate was set at 1.2 mL/min. The elution time for DTS was determined to be 27.438 minutes. Dutasteride's breakdown products were found when it was hydrolyzed in an acidic and alkaline solution. 1.0 mg/mL of 1M HCl was used to perform the acid and alkaline breakdown of DTS. The decomposition was conducted under reflux conditions at 80°C, with the acid decomposition lasting 8 hours and the alkaline decomposition lasting 4 hours.[35]
Kattempudi Paljashuva et. al. developed a stability-indicating RP-HPLC method for quantifying dutasteride in tablet dosage formsA Shimadzu HPLC system with an Agilent C18 column (250 x 4.6 mm, 5 µm) was used to achieve separation. Methanol and water were combined in the mobile phase in a 50:50 (v/v) ratio. At a flow rate of 1.0 mL/min, the analysis was carried out, and a PDA detector operating at 280 nm was used for detection. Dutasteride had a retention time of 2.623 minutes. The method demonstrated linearity for dutasteride concentrations ranging from 10 to 50 µg/mL, with a correlation coefficient of 0.999.[36]
Kudupudi Chandrasekhar et. al. developed a stability-indicating RP-HPLC method for quantifying armodafinil in tablet dosage forms. A Kromasil C18 column (250 x 4.6 mm, 5 µm) with a reverse phase isocratic elution was used to produce the separation. With a flow rate of 1.0 mL/min, the mobile phase was composed of 65% acetonitrile and 35% phosphate buffer. A PDA detector operating at 225 nm was used for the detection. The retention time of armodafinil was 12.914 minutes. The method exhibited linearity for armodafinil concentrations ranging from 25 to 75 µg/mL, with a correlation coefficient of 0.999. [37]
S.C. Rajesh et. al. outlined a stability-indicating RP-HPLC method for analyzing armodafinil in capsule dosage forms. Methanol and a 100 mM dipotassium hydrogen phosphate buffer (pH 9.5) were combined in a 20:80 (% v/v) ratio to form the mobile phase. The analysis was carried out using a Dionex C18 column (250 × 4.6 mm, 5 µm) with a 20 µL injection volume, and the flow rate was kept at 1.2 mL/min. A PDA detector was used to detect the signal at 295 nm, and the retention period was 2.880 minutes. The approach showed linearity with a correlation coefficient of 0.9993 for armodafinil concentrations between 50 and 150 µg/mL. The percentage recovery of armodafinil was found to be 98.53%.[38]
Devanna N et. al. developed a stability-indicating RP-HPLC method for quantifying dutasteride in tablet dosage forms. A 5 µm particle size, 250 mm × 4.6 mm Xterra C18 column was used for the RP-HPLC separation. Acetonitrile, phosphate buffer (pH 6.5), and water were combined in a 75:15:10 (v/v/v) ratio to create the mobile phase.
|
Sr. No |
Drug |
Matrix/ Dosage form |
Stationary Phase |
Mobile Phase |
Detection (nm) |
Flow Rate (ml/min) |
Retention Time (minutes) |
Detector |
Ref. No. |
|
1 |
Dutasteride |
Tablet |
C18 column (XTerra model, 4.6 x 250 mm, 5 μm) |
phosphate buffer with acetonitrile in a 40:80 (% v/v) ratio |
280 nm |
1.0 ml/min |
2.2 minutes |
DAD detector |
29 |
|
2 |
Dutasteride |
Tablet |
4.6 mm × 250 mm analytical column, BDS HyperSIL C18 |
Acetonitrile: Water: Methanol in a 75:10:15 (V/V/V) |
274 nm |
0.7 ml/min |
8.34 minutes |
UV detector |
30 |
|
3 |
Dutasteride |
Tablet |
C18 column (XTerra model, 4.6 × 150 mm, 5 µm) |
Phosphate buffer and acetonitrile in a 20:80 (v/v) ratio |
274 nm. |
0.8 ml/min |
2.003 minutes |
UV-PDA detector |
31 |
|
4 |
Dutasteride |
Tablet |
Column Phenomenex C18 (250 mm × 4.6 mm, particle size 5 μm) |
methanol and water in an 80:20 (v/v) ratio |
264 nm |
1.0 ml/min |
2.94 minutes |
UV detector |
32 |
|
5 |
Dutasteride |
Tablet |
5 µm particle size, 150 mm × 4.6 mm Kromasil C18 column |
Mixed phosphate buffer and acetonitrile in a 30:70 (% v/v) ratio |
242 nm. |
0.8 ml/min |
7.2 minutes |
UV detector |
33 |
|
6 |
Dutasteride |
Tablet |
XTerra C18 column (size: 150 x 4.6 mm; particle size: 5 µm) |
ammonium dihydrogen phosphate buffer (pH 6.5) and methanol in a 25:75 (% v/v) ratio |
246nm |
1.0 mL/min |
4.26 minutes |
UV detector |
34 |
|
7 |
Dutasteride |
Tablet |
3V column of inertsil ODS, C18 (150 x 4.6 ID) 5µm |
20 mM ammonium acetate buffer, acetonitrile, with methanol in a 40:30:30 (% v/v/v) ratio |
223 nm. |
1.0 ml/min |
- |
UV detector |
35 |
|
8 |
Dutasteride |
Tablet |
Shiseido C18 |
MeOH, ACN, and H2O in a 75:10:15 (v/v/v) ratio |
274 nm |
0.7 ml/min |
8.34 minutes |
UV detector |
36 |
|
9 |
Dutasteride |
Tablet |
Hi-Q Sil C18HS column |
ammonium acetate buffer (pH 4.5) and methanol in a 20:80 (v/v) ratio |
225 nm |
1.2 mL/min |
27.438 minutes |
UV detector |
37 |
|
10 |
Dutasteride |
Tablet |
Agilent C18 column (size: 250 x 4.6 mm; particle size: 5 µm) |
methanol and water in a 50:50 % (v/v) ratio |
280 nm |
1.0 mL/min |
2.623 minutes |
PDA detector |
38 |
|
11 |
Dutasteride |
Tablet |
Kromasil C18 column (250 × 4.6 mm, 5 µm particle size) |
phosphate buffer and acetonitrile in a 35:65 % (v/v) ratio |
225 nm |
1.0 mL/min. |
12.914 minutes |
PDA detector |
39 |
|
12 |
Dutasteride |
Capsule |
5 µm particle size in a 250 × 4.6 mm Dionex C18 column |
Methanol to buffer (100 mM dipotassium hydrogen phosphate) in a 20:80 % (v/v) ratio |
295 nm |
1.2 mL/min |
2.880 minutes |
PDA detector |
40 |
|
13 |
Dutasteride |
Tablet |
Column XterraC18 (250mmL×4.6mmI.D×5µ) |
acetonitrile: phosphate buffer (pH-6.5): water 75:15:10 % (v/v/v) |
245 nm |
0.8 ml/min |
4.3 minutes |
- |
41 |
|
14 |
Dutasteride |
Tablet |
Chromosil C18 column (size: 250 mm x 4.6 mm; particle size: 5 µm) |
methanol, acetonitrile, and 2% o-phosphoric acid in a 60:20:20 % (v/v/v) ratio |
234 nm |
1.0 mL/min |
5.19 minutes |
- |
42 |
|
15 |
Dutasteride |
Tablet |
Phenomenex C18 column (Torrance, CA, USA) |
Methanol and 0.02 M ammonium acetate buffer in an 85:15 % (v/v) ratio |
274 nm |
1.0 mL/min |
5.22 minutes |
PDA detector |
43 |
|
16 |
Dutasteride |
Tablet |
C18 column (250 x 4.6 mm, particle size: 5 µm) |
ACN and water in a 90:10 % (v/v) ratio |
292 nm |
1.0 mL/min |
2.8 minute |
PDA detector |
44 |
|
Sr. No. |
Drug |
Matrix/ Dosage Form |
Stationary Phase |
Mobile Phase |
Detection (nm) |
Rf |
Linearity Range |
Ref. No. |
|
1 |
Dutasteride |
Tablets |
plates backed with aluminum foil and covered in 0.2 mm silica gel G60F254 layers |
toluene, methanol, with triethylamine in a 9:1.5:1 % (v/v/v) ratio |
280 nm |
0.65 |
20-2000 ng/spot |
43 |
|
2 |
Dutasteride |
Capsule |
silica gel 60 F254 normal phase |
Toluene ,ethyl acetate with acetic acid 7:3:0.5 % (v/v/v) |
210 nm |
- |
50-500 µg mL-1 |
45 |
|
3 |
Dutasteride |
Tablets |
F254 silica gel 60 |
toluene, methanol, dichloromethane with triethylamine in a 6:1:1:0.6 % (v/v) ratio |
247 nm |
0.65 |
500–1000 ng per band |
46 |
|
4 |
Dutasteride |
Tablets |
Pre-coated aluminum plates with silica gel (G60F254) |
toluene, methanol with triethylamine in a 9:2:1 % (v/v/v) ratio |
274 nm |
0.71 |
200–3000 ng per spot |
47 |
|
5 |
Dutasteride |
Tablets |
aluminum plates covered with 60 F254 silica gel |
ethyl acetate, ethanol with ammonia in a 9:1:0.2 % (v/v/v) ratio |
254 nm |
0.78 |
3-35 mg/band |
48 |
|
Sr. No. |
Drug |
Matrix/ Dosage Form |
Stationary Phase |
Mobile Phase |
Detection (nm) |
Flow Rate (ml/min) |
Ret. Time (min.) |
Ref. No. |
|
1 |
Dutasteride |
Capsule |
C18 column, 150 mm by 4.6 mm |
acetonitrile and water combined in a 60:40 % (v/v) ratio |
210 nm |
1.0 mL/min. |
10 minutes |
49 |
|
2 |
Dutasteride |
Human plasma |
Hypurity C18 column with an internal diameter of 50 × 4.6 mm and a particle size of 5 µm |
10 mM ammonium formate with acetonitrile in a 20:80 % (v/v) ratio |
- |
0.6 mL/min |
2.5 minutes |
50 |
|
3 |
Dutasteride |
Human plasma |
50 mm × 2.0 mm, 3 µm particle size, Gemini C-18 column |
methanol with ammonium formate in a 97:3 (v/v) % ratio |
- |
1 mL/min |
0.39 minutes |
51 |
|
4 |
Dutasteride |
Human plasma |
Xterra MS C18 column |
10 mM ammonium formate and acetonitrile in a 15:85 (volume/volume) ratio |
- |
0.6 mL/min |
1.2-minute |
52 |
|
Sr. No. |
Drug |
Matrix/ Dosage Form |
Stationary Phase |
Carrier Gas |
Retention time (min.) |
Flow Rate |
Ref. No. |
|
1 |
Dutasteride |
Tablet |
DB-5MS column (phase composition: 5% phenyl-methylpolysiloxane ñ 95% phenyl arylene polymer) |
Nitrogen |
- |
- |
53 |
The separation was carried out at room temperature with a flow rate maintained at 0.8 mL/min. The retention period for dutasteride was 4.3 minutes. UV detection at 245 nm was used to quantify, and peak area analysis was used for the analysis. Linear calibration curves were established for dutasteride concentrations ranging from 10 to 50 µg/mL.[39]
P. Nagaraju et. al. developed a stability-indicating RP-HPLC method for quantifying dutasteride in tablet dosage forms. A Chromosil C18 column (250 mm × 4.6 mm, 5 µm) was used for the separation, and a UV detector was used for detection at 234 nm. Methanol, acetonitrile, and 2% o-phosphoric acid were combined in a 60:20:20 (v/v/v) ratio to form the mobile phase. Dutasteride had a 5.19-minute retention period, and the flow rate was kept at 1.0 mL/min. The calibration plots demonstrated a linear relationship (r = 0.999) for dutasteride concentrations ranging from 20 to 120 µg/mL.[40]
D.B. Patel et al. presented a validated stability-indicating HPLC method for quantifying dutasteride in pharmaceutical dosage forms. A Phenomenex (Torrance, CA, USA) C18 column was used for the HPLC process, and the mobile phase consisted of 15% ammonium acetate buffer (0.02 M) and 85% methanol (pH 9.5, corrected with triethylamine). Photodiode array (PDA) detection at 274 nm was used for quantification, with the flow rate set at 1.0 mL/min. Dutasteride had a 5.22 minute retention period. The method covered a concentration range of 1–20 μg/mL, with the mean recovery of dutasteride being 99.94 ± 0.611%.[41]
K. Vanitha Prakash et. al. dyeveloped a stability-indicating RP-HPLC method for measuring dutasteride in tablet formulations. The Waters LC setup (Waters, Milford, MA, USA) with a Waters C18 column (250 × 4.6 mm, 5 µm) was used by the HPLC system. The mobile phase used in the analysis was a 90:10 volume/volume combination of water and acetonitrile. A Waters model 2998 photodiode array detector and a Waters model 717 Plus auto sampler were also incorporated in the system. Empower Pro software (Waters, Milford, MA, USA) was used for data analysis. The analytes were found at 292 nm when the mobile phase was pumped at a flow rate of 1.0 mL/min. The method's linearity was evaluated for DTA within a concentration range of 50-150 µg/mL, with correlation coefficients of r = 0.9993 for DTA and r = 0.9997 for another analyte.[42]
HPTLC method
D.B. Patel et. al. described a stability-indicating HPTLC method for estimating dutasteride in bulk and tablet formulationsThe plates used in the stationary phase had aluminum foil backing and were covered in 0.2 mm layers of silica gel G60F254. Toluene, methanol, and triethylamine were combined in the mobile phase at a 9:1.5:1 (v/v/v) ratio. Densitometric scanning was conducted at 280 nm using a Camag TLC scanner III with a deuterium lamp. Dutasteride produced a distinct band with an Rf value of 0.65. A strong linear relationship (r² = 0.9954) was observed between the peak area and concentration over the range of 20-2000 ng/spot, as determined by linear regression analysis.[41]
S.S. Kamat et. al. detailed a stability-indicating HPTLC method for estimating dutasteride in bulk and capsule formulations. The analysis used silica gel 60 F254 as a normal phase stationary phase and toluene, ethyl acetate, and acetic acid in a 7:3:0.5 (v/v/v) ratio for the mobile phase. The UV wavelength used for detection was 210 nm. Calibration curves demonstrated linearity over the range of 50-500 µg/mL, with an R² value greater than 0.998. [43]
Sunil R. Dhaneshwar et. al. described a stability-indicating HPTLC method for quantifying dutasteride in bulk and tablet formulations. The mobile phase for the analysis consisted of toluene, methanol, dichloromethane, and triethylamine in a 6:1:1:0.6 (v/v) ratio. The stationary phase used in the analysis was silica gel 60 F254. At 247 nm, a densitometric analysis of the divided zones was carried out. Linear regression analysis (r² = 0.995) demonstrated good linearity across the concentration range of 500–1000 ng per band for dutasteride, with a retention factor (Rf) of 0.65.[44]
Dipti B. Patel et al. developed a stability-indicating HPTLC method for estimating dutasteride in bulk and tablet formulations. Utilizing aluminum plates precoated with silica gel G60F254 as the stationary phase, thin-layer chromatography was carried out. In a 9:2:1 (v/v/v) ratio, toluene, methanol, and triethylamine made up the solvent system. With an Rf value of 0.71 ± 0.01 for dutasteride, this approach generated separate spots. At 274 nm, densitometric measurement was carried out in absorbance mode.Linear regression analysis indicated excellent linearity (r² = 0.9989) for the peak area across the concentration range of 200–3000 ng per spot.[45]
Mina Wadie et. al. developed a stability-indicating HPTLC method to estimate dutasteride in both bulk and tablet forms. Normal HPTLC silica gel 60 F254 aluminum plates (Merck, Darmstadt, Germany) were used as the stationary phase in the chromatographic separation. The ratio of ethyl acetate to ethanol to ammonia was 9:1:0.2 in the mobile phase.Dutasteride had a retention factor (Rf) of 0.78. The intensities of the drug spots were measured using ImageJ software across concentration ranges from 3 to 35 mg per band. Densitometric analysis of dutasteride was carried out in absorbance mode at 254 nm.[46]
Liquid chromatography - Mass Spectrometry
Sudhir S. Kamat et. al. developed a stability-indicating LC–MS method for analyzing dutasteride in capsule formulations. A 150 mm x 4.6 mm C18 column was used in the chromatography, and a flow rate of 1.0 mL/min was employed in the mobile phase, which consisted of acetonitrile and water in a 60:40 (v/v) ratio. Dutasteride was detected using UV at 210 nm, with a retention time of approximately 10 minutes. The method demonstrated linearity over a concentration range of 0.2–1 µg/mL, with an R² value of 0.997.[47]
Noel A. Gomes et. al. developed a stability-indicating LC–MS method for analyzing dutasteride in human plasma. A Hypurity C18 column (50 x 4.6 mm i.d., 5 µm particle size) was used for the analysis. The mobile phase consisted of acetonitrile in a 20:80 (v/v) ratio, 10 mM ammonium formate buffer, and pH adjusted with formic acid to 3.00 ± 0.05. A constant flow rate of 0.6 mL/min was maintained.This method was validated for dutasteride concentrations ranging from 0.1 to 10.0 ng/mL and featured a rapid run time of 2.5 minutes, enabling the analysis of over 180 human plasma samples per day. [48]
Sangita Agarwal et. al. developed a stability-indicating LC–MS method for analyzing dutasteride in human plasma. Liquid-liquid extraction and chromatography on a Gemini C-18 column (50 mm x 2.0 mm, 3 µm) were the steps in the procedure. The ratio of methanol to ammonium formate in the mobile phase was 97:3 (v/v). a retention time of approximately 0.39 minutes.With a quick run time of just one minute per sample, the LC system operated isocratically at a flow rate of one milliliter per minute.The internal standard concentration in the plasma samples was 8.0 ng/mL.[49]
N.V.S. Ramakrishna et. al. described a method for evaluating the stability of dutasteride in pharmaceutical formulations using LC–MS with human plasmaThe procedure made use of a reverse phase Xterra MS C18 column with a mobile phase consisting of 10 mM ammonium formate and acetonitrile in a 15:85 (v/v) ratio. Formic acid was used to bring the pH down to 3.0. The assay demonstrated a linear dynamic range for dutasteride in human plasma, ranging from 0.1 to 25.0 ng/mL. With a rapid run time of 1.2 minutes per sample, the method enabled the analysis of more than 400 plasma samples daily, and a flow rate of 0.6 mL/min provided well-resolved peaks. [50]
Gas Chromatography-Mass Spectrometry
Aleksandra Groman et al. developed a direct standard headspace method using GC-MS to analyze benzene, carbon tetrachloride, and 1,2-dichloroethane in dutasteride drug substance. Nitrogen was used as the carrier gas at 60 kPa with a split ratio of 4:1 in a DB-5MS column containing 5% phenyl-methylpolysiloxane and 95% phenyl arylene polymer for the analysis. The gas chromatograph was injected with a 2 µL sample. Characteristic ions for detection were benzene at m/z 78, carbon tetrachloride at m/z 117, and 1,2-dichloroethane at m/z 62.[51]
Conclusion
Numerous methods can be employed to assess the presence of dutasteride in pharmaceutical formulations and biological materials. The study of the published data showed that the measurement of Dutasteride in different pharmaceutical dosage form, serum was frequently done using HPLC methods. Because this technology yields precise results and is less expensive than more sophisticated detection techniques, HPLC with UV detection is appropriate. This review provided a summary of the most cutting-edge analytical techniques available for identifying Dutasteride.
This review will be valuable to analytical chemists by providing insights into the essential solvents and their combinations for the analytical tools available in the laboratory. Utilizing the most effective set of parameters can reduce both the time and cost of analyses while ensuring reliable results. Additionally, these techniques will aid in selecting optimal parameters for in-process analysis during API production.
Source of Funding
None.
Conflict of Interest
None.
References
- Sweetman S. . The Complete Drug Reference. 2005. [Google Scholar]
- Budavari S. . The Merck Index:. 2001. [Google Scholar]
- Debruyne F, Barkin J, Erps P, Reis M, Tammela T, Roehrborn C. Efficacy and safety of long-term treatment with the dual 5α-reductase inhibitor dutasteride in men with symptomatic benign prostatic hyperplasia. Eur Urol. 2004;46(4):488-95. [Google Scholar]
- Keam SJ, Scott L. Dutasteride: a review of its use in the management of prostate disorders. Drugs. 2008;68:463-85. [Google Scholar]
- Varshini C, Kumar K, Sushma S, Prakash K. Development and validation of RP-HPLC method for simultaneous estimation of Alfuzosin Hydrochloride and Dutasteride in bulk and pharmaceutical dosage form. Inventi J. 2012;2012(4):1-4. [Google Scholar]
- Patel D, Patel N, Patel S, Prajapati A, Patel S. RP-HPLC method for the estimation of dutasteride in tablet dosage form. Indian J Pharm Sci. 2010;72(1):113-6. [Google Scholar]
- Patel D, Patel N. Validated reversed-phase high-performance liquid chromatographic and high-performance thin-layer chromatographic methods for simultaneous analysis of tamsulosin hydrochloride and dutasteride in pharmaceutical dosage forms. Acta Chromatograph. 2010;22(3):419-31. [Google Scholar]
- Aines K. Dutasteride (Avodart): new 5-alphareductase inhibitor for treating BPH. Urologic Nurs. 2003;23(3):218-20. [Google Scholar]
- Brown C, Nuttall M. Dutasteride: a new 5-alpha reductase inhibitor for men with lower urinary tract symptoms secondary to benign prostatic hyperplasia. Int J Clin Pract. 2003;57(8):705-9. [Google Scholar]
- Gisleskog P, Hermann D, Udenaes M, Karlsson M. The pharmacokinetic modelling of GI198745 (dutasteride), a compound with parallel linear and nonlinear elimination. Brit J Clin Pharm. 1999;47(1):53-8. [Google Scholar]
- Tian G, Mook R, Moss M, Frye S. Mechanism of time-dependent inhibition of 5. alpha.-reductases by. delta. 1-4-azasteroids: toward perfection of rates of time-dependent inhibition by using ligand-binding energies. Biochem. 1995;34(41):13453-9. [Google Scholar]
- Clark R, Matsumoto A. Bone density, bone metabolism markers and lipid profiles in healthy men are unaffected by the novel dual 5A-reductase inhibitor dutasteride. J Urol. 2003;169(4). [Google Scholar]
- Bramson H, Hermann D, Batchelor K, Lee F, James M, Frye S. Unique preclinical characteristics of GG745, a potent dual inhibitor of 5AR. J Pharma Exp Therap. 1997;282(3):1496-502. [Google Scholar]
- Critchley P, Brin S, Komas B. Dutasteride: clinical investigators brochure (US). Triangle Park (NC): GlaxoSmithKline.. . 2002. [Google Scholar]
- Roehrborn C, Andriole G, Nickel C. Efficacy and safety of a dual inhibitor of 5-alpha-reductase types 1 and 2 (dutasteride) in men with benign prostatic hyperplasia. Urology. 2002;60(3):266-73. [Google Scholar]
- Bramson H, Hermann D, Batchelor K, Lee F, James M, Frye S. Unique preclinical characteristics of GG745, a potent dual inhibitor of 5AR. J Pharmacol Exp Ther. 1997;282(3):1496-502. [Google Scholar]
- Clark RV, Hermann DJ, Gabriel H. Almost complete suppression of dihydrotestosterone in men with benign prostatic hyperplasia by GI198745, a novel, dual 5alpha reductase inhibitor. North Carolina: GlaxoSmithKline.. . . [Google Scholar]
- Roehrborn C, Boyle P, Nickel J. Efficacy and safety of a dual inhibitor of 5-alpha-reductase types 1 and 2 (dutasteride) in men with benign prostatic hyperplasia. Urology. 2002;60(3):434-41. [Google Scholar]
- Andriole G, Roehrborn C, Nickel C. Effect of the dual 5alpha-reductase inhibitor dutasteride on markers of tumor regression in prostate cancer. J Urol. 2002;172(3):915-9. [Google Scholar]
- . Prescribing information. Avodart (dutasteride). Research Triangle Park, NC: GlaxoSmithKline. . 2005. [Google Scholar]
- . GlaxoSmithKline. Avodart: Prescribing information. North Carolina: GlaxoSmithKline. . 2002. [Google Scholar]
- Pohlman G, Pohlman E, Crawford E. Dutasteride: a review of its use in the management of prostate disorders. Clin Med. 2011;3. [Google Scholar]
- Evans H, Goa K. Dutasteride. Drugs Aging. 2003;20(12):905-16. [Google Scholar]
- Hasanuzzaman M, Islam MK. Validated uv spectrophotometric method for estimation of dutasteride in tablet dosage form. Int J Comp Pharm. 2011;1(4):1-3. [Google Scholar]
- Ali M, Juyal D, Singh A. UV Spectrophotometric Method Development for the Estimation of Dutasteride in Bulk Drugs and Pharmaceutical Dosage Form. Ally J Pharm Sci. 2014;1(1):1-11. [Google Scholar]
- Choudhari V, Gite S, Raut R, Hable A, Parekar S, Kuchekar B. Spectrophotometric simultaneous determination of dutasteride and tamsulosin in combined tablet dosage form by first order derivative spectroscopy and area under curve (AUC) spectrophotometric methods and its application to uniformity of content in tablet and capsule. Int J Pharm Sci. 2010;2(2):63-7. [Google Scholar]
- Kumar A, Saradhi S, Sekaran C, Reddy T, Kuppuswamy J. Spectrophotometric analysis of dutasteride in pure and tablet dosage forms. Chem Sci J CSJ. 2012;47:1-20. [Google Scholar]
- Al-Bayatie Y, Muhamad Y, Kareem Z, Khathi M. Determination of dutasteride in pure and tablet by flow injection system. Int J Health Sci. 2022;6(3):201-8. [Google Scholar]
- Prasad Y. Determination of dutasteride for analytical method development and validation in bulk as well as in pharmaceutical dosage form by using RP. World J Pharm Sci. 2022;10(9):61-9. [Google Scholar]
- Deshmukh A, Shirode M, Kadam V. Analytical Method Development and Validation for the Quantitative Estimation of Dutasteride in Its Tablet Dosage Form by RP-HPLC Method. Int J Creat Res Thoughts . 2021;9(6):120-31. [Google Scholar]
- Reddy G, Kumar S, Debnath M, Kumar V. Stability indicating RP-HPLC method development & validation for simultaneous determination of dutasteride and tamsulosin in bulk as well as in pharmaceutical dosage form by using PDA detector. Asian J Pharm Clin Res. 2014;7(2):105-13. [Google Scholar]
- Satpute P, Jadhav S, Rathod M, Naykodi P. RP-HPLC Forced Degradation Study of Alfuzosin and Dutasteride. Pharm Res. 2018;1(1):21-5. [Google Scholar]
- Diyya K. Novel stability indicating rp-hplc method development and validation for simultaneous estimation of alfuzosin and dutasteride in pharmaceutical dosage form. Indian J Pharm Educ Res. 2020;54(4):1144-52. [Google Scholar]
- Ahmed S, Karunakranth D, Babu R, Khasim S, Khan M, Ghouse S. To develop new RP HPLC method for the simultaneous estimation of tamsulosin hydrochloride and dutasteride in pharmaceutical dosage form. J Drug Dev Del. 2019;2:7-12. [Google Scholar]
- Chaudhari R, Mohanraj K, Shirsat V. MS/MS and HPLC characterization of forced degradation products of dutasteride and tamsulosin hydrochloride. Int J Pharm Sci Res. 2014;5(7). [Google Scholar]
- Paljashuva K, Ramarao N. Simultaneous estimation of dutasteride and silodosin in bulk form by RP-HPLC method. World J Curr Med Pharm Res. 2019;1(5):148-63. [Google Scholar]
- Chandrasekhar K, Manikandan A. Novel RP-HPLC method development and validation of Tamsulosin hcl and dutasteride in tablets by ratio’s method. Rasayan J Chem. 2021;14(2):665-71. [Google Scholar]
- Rajesh S, Neelima K, Solairaj P. Method development and validation for the simultaneous estimation of dutasteride and tamsulosin hydrochloride in capsule dosage form by rp-hplc. W J Pharm Res. 2014;3(1):535-47. [Google Scholar]
- Madhusudhan P, Reddy M, Devanna N. Method development and validation of Alfuzosin HCl and Dutasteride in pharmaceutical dosage form by RP-HPLC. Int J Novel Tends PharmaSci. 2015;5(3):94-101. [Google Scholar]
- Nagaraju P, Prasad B, Priyadarshini G. Development and validation of a Reversed Phase HPLC method for simultaneous determination of Tamsulosin and Dutasteride in tablet dosage form. Adv Pharm J. 2017;2(4):134-8. [Google Scholar]
- Patel D, Patel N. Validated reversed-phase high-performance liquid chromatographic and high-performance thin-layer chromatographic methods for simultaneous analysis of tamsulosin hydrochloride and dutasteride in pharmaceutical dosage forms. Acta Chromatographica. 2010;22(3):419-31. [Google Scholar]
- Ishaq B, Prakash K, Mohan G. Simultaneous determination of dutasteride and tamsulosin in pharmaceutical dosage forms by RP-HPLC. Int Confer Harmonization (ICH) Anal Method Val Guid. 2014;6(3):103-9. [Google Scholar]
- Kamat S, Vele V, Choudhari V, Prabhune S. Determination of dutasteride from its bulk drug and pharmaceutical preparations by high performance thin layer chromatography. Asian J Chem. 2008;20(7):5514-8. [Google Scholar]
- Deshmukh S, Musale V, Bhusari V, Dhaneshwar S. Validated HPTLC method for simultaneous analysis of alfuzosin hydrochloride and dutasteride in a pharmaceutical dosage form. JPC-J Planar Chromatography-Modern TLC. 2011;24(3):218-21. [Google Scholar]
- Patel D, Patel N, Patel S, Patel P. Validated stability indicating HPTLC method for the determination of dutasteride in pharmaceutical dosage forms. Chromatography Res Int. 2011;278923:1-5. [Google Scholar]
- Wadie M, Abdel-Moety E, Rezk M, Marzouk H. Smartphone-based high-performance thin Layer chromatographic method along with benchtop densitometry for simultaneous quantification of co-formulated dutasteride with silodosin and their residuals on manufacturing equipment's surfaces. Bull Fac Pharm Cairo Univ. 2023;61(1):1-4. [Google Scholar]
- Kamat S, Choudhari V, Vele V, Prabhune S. Determination of dutasteride by LC: validation and application of the method. Chromatographia. 2008;67(11):911-6. [Google Scholar]
- Gomes N, Pudage A, Joshi S, Vaidya V, Parekh S, Tamhankar A. Rapid and sensitive LC-MS-MS method for the simultaneous estimation of alfuzosin and dutasteride in human plasma. Chromatographia. 2009;69:9-18. [Google Scholar]
- Agarwal S, Gowda K, Sarkar A, Ghosh D, Bhaumik U, Chattaraj T. Simultaneous determination of tamsulosin and dutasteride in human plasma by LC-MS-MS. Chromatographia. 2008;67:893-903. [Google Scholar]
- Ramakrishna N. Selective and rapid liquid chromatography-tandem mass spectrometry assay of dutasteride in human plasma. J Chromatog B. 2004;1:117-24. [Google Scholar]
- Groman A, Stolarczyk S, Lipiec-Abramska E. Development and validation of gas chromatography methods for the control of volatile impurities in the pharmaceutical substance dutasteride. Acta Pol Pharm Drug Res. 2017;74:1343-51. [Google Scholar]
How to Cite This Article
Vancouver
Jain PS, Chaudhari H, Chaudhari M, Haswani N. A concise review- Development of an analytical method and validation of dutasteride [Internet]. Curr Trends Pharm Pharm Chem. 2024 [cited 2025 Oct 09];6(4):180-192. Available from: https://doi.org/10.18231/j.ctppc.2024.034
APA
Jain, P. S., Chaudhari, H., Chaudhari, M., Haswani, N. (2024). A concise review- Development of an analytical method and validation of dutasteride. Curr Trends Pharm Pharm Chem, 6(4), 180-192. https://doi.org/10.18231/j.ctppc.2024.034
MLA
Jain, Pritam S, Chaudhari, Hina, Chaudhari, Mayuri, Haswani, NG. "A concise review- Development of an analytical method and validation of dutasteride." Curr Trends Pharm Pharm Chem, vol. 6, no. 4, 2024, pp. 180-192. https://doi.org/10.18231/j.ctppc.2024.034
Chicago
Jain, P. S., Chaudhari, H., Chaudhari, M., Haswani, N.. "A concise review- Development of an analytical method and validation of dutasteride." Curr Trends Pharm Pharm Chem 6, no. 4 (2024): 180-192. https://doi.org/10.18231/j.ctppc.2024.034
