- Visibility 179 Views
- Downloads 18 Downloads
- DOI 10.18231/j.ctppc.2021.007
-
CrossMark
- Citation
Microwave assested synthesis of phthalimide amino derivatives with their antioxidant potential
- Author Details:
-
Ajay Singh Bisht *
-
Rajat Bisht
Introduction
Imide is the form of amide in which the nitrogen atom is affix to two carbonyl group. Imide mention to any compound which contains the divalent radical. These compounds are obtained from ammonia or primary amine, where two hydrogen atoms are replaced by a bivalent acid group or two monovalent acid groups, resulting in comprising of two carboxylic acid groups.[1]
Phthalimide possess a structural feature C8H5NO2 and an imide ring which help them to be biologically active and pharmaceutically useful.[2] Among bicyclic non-aromatic nitrogen heterocycles, phthalimides are an interesting class of compounds. Phthalimides have served as starting materials and intermediates for the synthesis of many types of alkaloids and pharmacophores.[3] The structural diversity and biological importance of nitrogen containing heterocycles have prepared them important targets for synthesis over many years.[4] Phthalimide and some of its derivatives proved to have received awareness due to their antibacterial, antifungal, analgesic, antitumour, anxiolytic and anti-HIV-1 activities.When phthalimide is subjected to Mannich condensation, it may yield Mannich bases which may display more potent biological activities. The present research focuses on novel synthesized phthalimides having significant biological activities.[3] Amongstheterocyclic scaffolds, phthalimides are of particular biological interest and have been reported as herbicides, insecticides and anti-inflammatory agents. Phthalimide are an important class of drugs exhibiting anxiolytic, antimicrobial, antibacterial, antituberculosis, anticancer, hypolipidemic, analgesic, antiproliferative, acetylcholinesterase inhibitors and inhibitor of human neuronal nitric oxide synthase.[4] Chemically, oxidation is the removal of electrons and reduction is the gain of electrons, oxidation is always coupled with reduction. Many biological oxidation can takes place without the participation of molecular oxygen, e.g. dehydration.[5] “Antioxidants are reducing agents which are add up to the drug or other pharmaceuticals to avert their oxidation through oxidative process”.[6] For convenience, antioxidants have been traditionally divided into two classes, primary or chain breaking antioxidants and secondary or preventative antioxidants. Secondary or preventative antioxidants are compounds that retard the rate of oxidation.[7] Many human diseases and degeneration process have been linked in some way to the action of free radicals.[6], [7], [8]
The aim of the present study is to synthesize Phthalimide derivatives by using microwave assisted synthesis method and compare the activity of the synthesized molecules.
Materials and Methods
Phthalic anhydride, Urea, Glycine, Sulphanilic acid, Aniline, Chloroform & DPPH were received from Central Drug House Ltd. New Delhi, India. Methanol, Ethyl acetate & n-Hexane were received from Himedia Pvt. Ltd.
Method and methodology
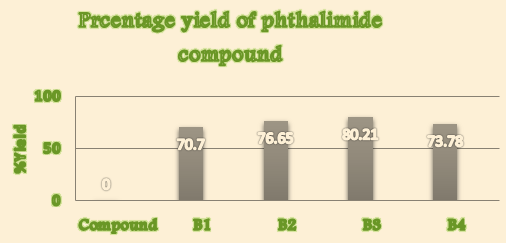
Thus, the present communication utilized the technology gracefully for the synthesis, identification and characterization of some novel derivatives by the reaction of Phthalic anhydride with urea, glycine, aniline, sulphanilic acid to yield various Phthalimide derivatives using domestic microwave by getting percentage yield 70.7%, 76.65%, 80.21% and 73.78% of synthesized compound B1,B2,B3 and B4 respectively. All synthesized compound(s) were subjected to melting point determination, TLC analysis, column chromatography (for purification), 1H-NMR and Mass Spectrometry.
Result and Discussion
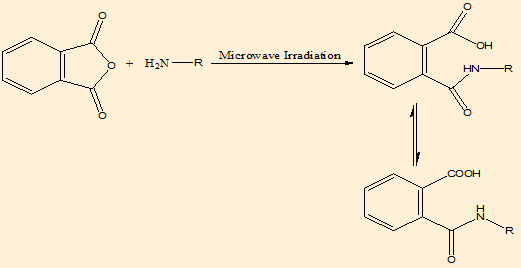
We developed new synthetic methodologies for the synthesis of Phthalimidederivatives. The starting material phthalic acid was reacted with amino acids to give phthalimide, the addition reaction was takes place:
Mechanism of synthesis([Figure 1])
For phthalimide derivatives
Percentage yield([Table 1])
|
S.No. |
Compounds |
% yield |
|
1. |
B1 |
70.7 |
|
2. |
B2 |
76.65 |
|
3. |
B3 |
80.21 |
|
4. |
B4 |
73.78 |
Physicochemical properties([Table 2])
|
Compound |
Molecular formula |
Molecular weight |
Appearance |
Percentage yield (%) |
|
B1 |
C9H6O3N2 |
190.16 |
White crystals |
70.70 |
|
B2 |
C10H7O4N |
205.17 |
Brown powder |
76.65 |
|
B3 |
C14H9O2N |
223.23 |
Off White crystals |
80.21 |
|
B4 |
C14H9O5NS |
303.29 |
Off White crystals |
73.78 |
Melting point([Table 3])
|
S.No. |
Compound |
Melting Point Range ( 0 C) |
|
1. |
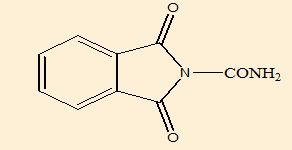
1, 3-dioxoisoindoline-2-carboxamide (B1) |
210±2 |
|
2. |
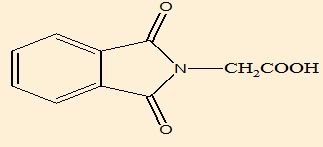
2-(1,3-dioxoisoindoline-2-yl)acetic acid (B2) |
146±2 |
|
3. |
2-phenylisoindoline-1,3-dione (B3) |
203±2 |
|
4. |
4-(1,3-dioxoisoindoline-2-yl)benzenesulfonic acid (B4) |
230±2 |
Solubility([Table 4])
|
S.No. |
Compound |
Solubility |
|
1. |
1, 3-dioxoisoindoline-2-carboxamide (B1) |
Water, Methanol |
|
2. |
2-(1,3-dioxoisoindoline-2-yl)acetic acid (B2) |
Water, Ethanol |
|
3. |
2-phenylisoindoline-1,3-dione (B3) |
Water, Chloroform |
|
4. |
4-(1,3-dioxoisoindoline-2-yl)benzenesulfonic acid (B4) |
Water, Ethanol |
PH([Table 5])
|
S.No. |
Compound |
Observation |
|
1. |
1, 3-dioxoisoindoline-2-carboxamide (B1) |
7.88 |
|
2. |
2-(1,3-dioxoisoindoline-2-yl)acetic acid (B2) |
11.53 |
|
3. |
2-phenylisoindoline-1,3-dione (B3) |
8.78 |
|
4. |
4-(1,3-dioxoisoindoline-2-yl)benzenesulfonic acid (B4) |
8.25 |
Ultraviolet spectroscopy([Table 6])
|
S.No. |
Compound |
Solvent |
Wavelength(nm) |
|
1. |
1, 3-dioxoisoindoline-2-carboxamide (B1) |
Water |
281nm |
|
2. |
2-(1,3-dioxoisoindoline-2-yl)acetic acid (B2) |
Water |
374nm |
|
3. |
2-phenylisoindoline-1,3-dione (B3) |
Water |
298nm |
|
4. |
4-(1,3-dioxoisoindoline-2-yl)benzenesulfonic acid (B4) |
Water |
245nm |
Thin layer chromatography([Figure 3])

|
S.No |
Compound |
Rf value |
|
1. |
1, 3-dioxoisoindoline-2-carboxamide (B1) |
0.70 |
|
2. |
2-(1,3-dioxoisoindoline-2-yl)acetic acid (B2) |
0.48 |
|
3. |
2-phenylisoindoline-1,3-dione (B3) |
0.60 |
|
4. |
4-(1,3-dioxoisoindoline-2-yl)benzenesulfonic acid(B4) |
0.52 |
The characteristic 1H NMR data and interpretation of synthesized compounds
|
Compound No. |
δ (ppm) |
Group |
No. of H |
|
B1 |
7.55-7.69 |
Ar-H |
4 |
|
B2 |
7.81- 7.94 4.32 |
Ar-H CH2 |
4 2 |
|
B3 |
7.41-7.54 7.88-7.97 |
Ar-H Ar-H |
5 4 |
|
B4 |
2.52-2.53 7.55-7.69 11.91 |
OH (Alcohol) Ar-H CH (Benzene) |
1 4 1 |
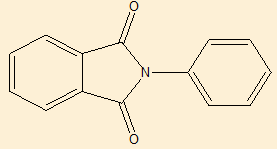
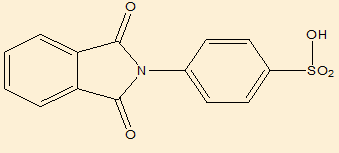
Elemental analysis([Table 9])
|
Comp. |
Mol. Formula |
Mol. Weight |
Compound- Name |
Calculated % found |
||||
|
C% |
H% |
N% |
O% |
S |
||||
|
B1 |
C9H6O3N2 |
190.16 |
1, 3-dioxoisoindoline-2-carboxamide (B1) |
56.85% |
03.18% |
14.73% |
25.24% |
_ |
|
B2 |
C10H7O4N |
205.17 |
2-(1,3-dioxoisoindoline-2-yl)acetic acid (B2) |
58.54% |
03.44% |
06.83% |
31.19% |
_ |
|
B3 |
C14H9O2N |
223.23 |
2-phenylisoindoline-1,3-dione (B3) |
75.33% |
04.06% |
06.27% |
14.33% |
_ |
|
B4 |
C14H9O5NS |
303.29 |
4-(1,3-dioxoisoindoline-2-yl)benzenesulfonic acid (B4) |
49.52% |
13.36% |
04.12% |
23.56% |
09.44% |
Antioxidant activity([Table 10])
• In vito antioxidant activity by using DPPH scavenging method
|
Concentration (mg/ml) |
% inhibition |
||||
|
B1 |
B2 |
B3 |
B4 |
Ascorbic acid |
|
|
0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
|
0.02 |
13.35 |
31.23 |
16.88 |
65.72 |
84.02 |
|
0.04 |
17.51 |
44.95 |
21.26 |
67.43 |
86.35 |
|
0.06 |
18.88 |
53.93 |
24.37 |
69.09 |
86.57 |
|
0.08 |
20.92 |
55.72 |
24.77 |
69.52 |
86.92 |
|
0.10 |
25.22 |
59.79 |
25.59 |
69.56 |
98.42 |
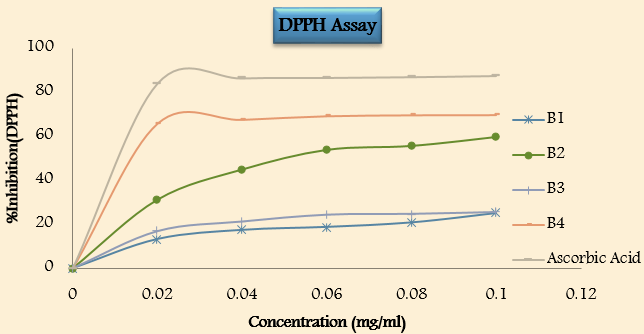
Conclusion
On the basis of the previous results, the study concluded that the synthesis of the designed compound has been successfully achieved. Purity and characterization of the synthesized compounds were confirmed by determination of physical properties (melting point, pH and Rf values), elemental analysis and 1H-NMR spectra. The compound B3 of phthalimide shows higher percentage practical yield. The in-vitro antioxidant activity of all synthesized compound was tested by using DDPH scavenging activity. The compounds were concealed at distinct concentration from 0.02-0.10mg/ml in order to examine the percentage inhibition of compounds. From the result in table (10) compound B4manifest highly significant activity against DPPH. The result shows that as the concentration of compound increase, the compound showed high remarkable activity against DPPH. The other complete tested compound showed low to moderate activity.Thus, the study could be concluded as the compounds have significant antioxidant activity. Thus, it may be concluded that the synthesized compound productively can be further used in the treatment of above-mentioned element.
Acknowledgement
The authors are thankful to the Himalayan Institute of Pharmacy & Research, Dehradun, Uttarakhand, India for helping us to carry out the study. We are thankful to the Uttarakhand technical university for their support in characterization studies. All the teaching staff of Himalayan Institute of Pharmacy & Research, Dehradun for their silent contribution in particular.
Source of Funding
None.
Conflict of Interest
None.
References
- M P Cava. N-PhenlyPhthalimide” Organic synthesis. 1973. [Google Scholar]
- N Kushwaha, D Kaushik. Recent Advances and Future Prospects of Phthalimide Derivatives. J Appl Pharm Sci 2016. [Google Scholar] [Crossref]
- Ashif, J Sharma, G J Baranwal, S Ahmad, M S Alam. Phthalimides: Biological Profile and Recent Advancements. Res J Pharm Technol 2013. [Google Scholar] [Crossref]
- S K Chimatahalli. Investigation of Antioxidant Properties of Phthalimide Derivatives. Borderless Sci publishing 2015. [Google Scholar] [Crossref]
- R S Satoskar, N Rege, S D Bhandarkar. Pharmacology and pharmacotherapeutics” Popular prakashan. 2009. [Google Scholar]
- D Prakash. Pharmaceutical inorganic chemistry” Pragati prakashan,. 2010. [Google Scholar]
- M Antolovich, P D Prenzler, E Patsalides, S McDonald, K Robards. Methods for testing antioxidant activity. Analyst 2002. [Google Scholar] [Crossref]
- Y Shebis, D Iluz, Y K Tahan, Z Dubinsky, Y Yehoshua. Natural Antioxidants: Function and Sources. Food Nutr Sci 2013. [Google Scholar] [Crossref]
- Introduction
- Materials and Methods
- Result and Discussion
- Mechanism of synthesis([Figure 1])
- For phthalimide derivatives
- Percentage yield([Table 1])
- Physicochemical properties([Table 2])
- Melting point([Table 3])
- Solubility([Table 4])
- PH([Table 5])
- Ultraviolet spectroscopy([Table 6])
- Thin layer chromatography([Figure 3])
- The characteristic 1H NMR data and interpretation of synthesized compounds
- Elemental analysis([Table 9])
- Antioxidant activity([Table 10])
- Conclusion
- Acknowledgement
- Source of Funding
- Conflict of Interest
How to Cite This Article
Vancouver
Bisht AS, Bisht R. Microwave assested synthesis of phthalimide amino derivatives with their antioxidant potential [Internet]. Curr Trends Pharm Pharm Chem. 2025 [cited 2025 Sep 07];3(3):23-27. Available from: https://doi.org/10.18231/j.ctppc.2021.007
APA
Bisht, A. S., Bisht, R. (2025). Microwave assested synthesis of phthalimide amino derivatives with their antioxidant potential. Curr Trends Pharm Pharm Chem, 3(3), 23-27. https://doi.org/10.18231/j.ctppc.2021.007
MLA
Bisht, Ajay Singh, Bisht, Rajat. "Microwave assested synthesis of phthalimide amino derivatives with their antioxidant potential." Curr Trends Pharm Pharm Chem, vol. 3, no. 3, 2025, pp. 23-27. https://doi.org/10.18231/j.ctppc.2021.007
Chicago
Bisht, A. S., Bisht, R.. "Microwave assested synthesis of phthalimide amino derivatives with their antioxidant potential." Curr Trends Pharm Pharm Chem 3, no. 3 (2025): 23-27. https://doi.org/10.18231/j.ctppc.2021.007
