Introduction
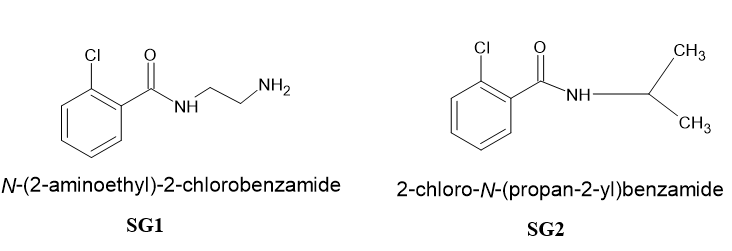
Benzamide derivatives are known for its versatile medicinal properties.1 Some of the pharmacological properties of benzamide derivatives include antipsychotic, 2 antihypertensive, 3 antibacterial4 and antimicrobial 5 properties. The structure of the claimed compounds has been shown in Figure 1. The synthesis of benzamides have been reported by many authors.6
Materials and Methods
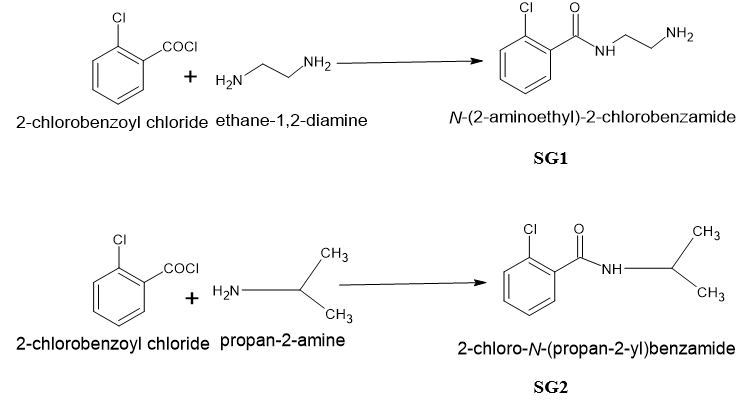
TLC was performed on 524nm Merk TLC plates. All chemicals were of synthetic grade and 98% purisis grade. TLC was eluted with 3 different solvents to check the purity of the compounds and visualized in Iodine chamber and further in UV chamber. The 1H-NMR was performed on Bruker 400 MHZ NMR before which FT-IR was performed on Perkin Elmer spectrophotometer. The synthetic scheme for the claimed compounds has been shown in Figure 2.
Synthetic scheme
N-(2aminoethyl)-2-chlorobenzamide (SG1): An equimolar solution of ethylene diamine was dissolved in 10 ml of ethanolic 1 N NaOH in round bottom flask and to it 2-Chlorobenzoyl chloride was added dropwise from dropping funnel with continuous stirring for 3 hrs at room temperature. The stirring was conducted on magnetic stirrer with magnetic bead in the ethylene diamine solution. The compound that separated out after 3 hrs was dried. The compound SG1 was washed with ethanol and further dried again washed with NaOH and water and air dried.
FT-IR (λ, cm-1): 3439.6, 3102.9, 3097.8, 3023.5, 2952.4, 1718.8, 1584.8, 1566.3, 1489.0, 1486.2, 1398.6, 1239.6, 1222.2, 1192.9, 1171.1, 929.0, 893.0, 884.5, 1222.2, 1192.9, 11717.1, 929.0, 893.0, 884.5, 786.6, 712.5, 697.0
1HNMR (δ shift in ppm): 2.83 (2H, t, J = 7.2 Hz), 3.47 (2H, t, J = 7.2 Hz), 7.32-7.59 (3H, 7.39 (ddd, J = 8.1, 7.6, 1.4 Hz), 7.51 (ddd, J = 8.4, 7.6, 1.5 Hz), 7.53 (ddd, J = 8.4, 1.4, 0.5 Hz)), 7.90 (1H, ddd, J = 8.1, 1.5, 0.5 Hz)
2-chloro-N-(propan-2-yl) benzamide (SG2): The procedure for the SG1 was repeated and in place of ethylene diamine, isopropyl amine was used. Rest of the procedure remains same.
FT-IR (λ, cm-1): 3459.5, 3436.1, 3384.5, 3114.3, 3098.6, 30882, 3076.0 , 2934.3 , 1743.0, 1584.3, 1570.5, 1551.5, 1448.0, 1450.0, 1492.2, 1149.2, 1072.1, 1023.3, 939.6.
1H-NMR (δ shift in ppm): 1.17 (6H, d, J = 6.8 Hz), 4.20 (1H, sept, J = 6.8 Hz), 7.32-7.59 (3H, 7.39 (ddd, J = 8.1, 7.6, 1.4 Hz), 7.51 (ddd, J = 8.4, 7.6, 1.5 Hz), 7.53 (ddd, J = 8.4, 1.4, 0.5 Hz)), 7.90 (1H, ddd, J = 8.1, 1.5, 0.5 Hz).
Results and Discussion
The compounds complied with IR and NMR spectral data and confirmed to be syhthesized.