Background
Pazopanib is a potent tyrosine kinase inhibitor targeting multiple pathways crucial in cancer progression, particularly for renal cell carcinoma and soft tissue sarcoma. However, its therapeutic application is hindered by poor solubility and bioavailability, necessitating high doses that increase systemic toxicity. Nanotechnology offers a promising solution to these limitations by enabling targeted drug delivery systems, such as nanoparticles, that enhance solubility, prolong release, and target specific tissues more effectively.
Nanoparticle drug delivery systems
Poly(DL-lactide-co-glycolide) (PLGA) is a biocompatible and biodegradable polymer widely used in drug delivery systems, allowing for sustained and targeted drug release. Formulating Pazopanib with PLGA into nanoparticles (NPs) could potentially optimize therapeutic outcomes by enhancing drug bioavailability, reducing toxicity, and prolonging release.1, 2, 3
Objective
This study aims to develop, characterize, and evaluate the in vitro release profile of Pazopanib-loaded PLGA nanoparticles to establish a more efficient therapeutic delivery method.
Materials and Methods
Materials
Drug: Pazopanib (GlaxoSmithKline Pharmaceuticals Ltd)
Polymer: PLGA (Sigma Aldrich)
Solvents and Reagents: DMSO, Acetone, Sodium Hydroxide, Deionized water
Preparation of pazopanib-loaded PLGA nanoparticles
The nanoparticles were prepared using the oil-in-water (o/w) emulsion solvent evaporation method. The drug and polymer were dissolved in an organic phase consisting of DMSO and Acetone. The organic phase was added drop wise into an aqueous phase with continuous stirring, and the mixture was subjected to overnight stirring to allow solvent evaporation. Nanoparticles were isolated via centrifugation, washed, and resuspended in saline.4
Table 0
Characterization
Particle size and zeta potential
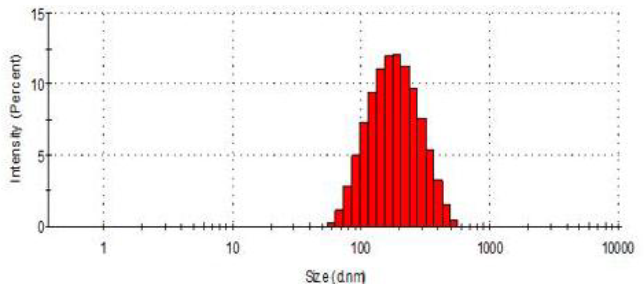
Particle size of nanoparticles (in nm) was determined using principle of dynamic light scattering (DLS). Light source was 633 nm He-Ne laser and scattering angle was 90° (Nano ZS90, Malvern Instruments Ltd., UK, Zeta Sizer Software Ver. 7.10). Analysis was carried out in triplicate at 25 °C temperature after appropriate dilution with double distilled water. The results were reported as Z-average along with polydispersity index (PDI). 5, 6, 7, 8, 9
Zeta potential values were obtained using Smoluchowski equation which takes into account electrophoretic mobility of the particles and 90° back-scatter (Patil et al., 2016). The nanoparticles were sufficiently diluted with double distilled water and analysis was carried out in triplicate using zeta cuvette and ZetaSizer NanoZS 90 (Malvern Instruments Ltd., UK, Zeta Sizer software Ver. 7.10).
Morphology
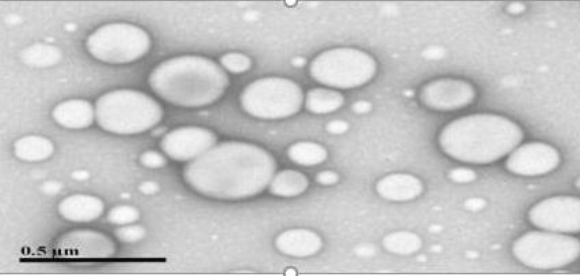
Evaluated via transmission electron microscopy (TEM) to confirm particle shape and distribution. Morphology, size, and shape of the PZ loaded nanoparticle were ascertained by transmission electron microscope (TEM) (JEOL JEM 1400 electron microscope with Gaton camera, Peabody, MA, USA). Sufficient amount of nanoparticle sample was placed on EMS formvar support film square grid, 200 Cu and allowed to air dry for 10 min. Subsequently, it was stained with 2% w/v phosphotungstic acid. The samples were examined at accelerating voltage of about 120 kV with 40,000 magnifications.
Encapsulation efficiency
For determination of entrapment efficiency, the reconstituted nanoparticles were treated with methanol to extract the loaded drug and were analysed by high performance liquid chromatography (HPLC) (Agilent 1100 HPLC System) 260 nm (λmax) using C18 column. The mobile phase was 45% ammonium acetate with pH7, 55% of organic solvent is mixture of methanol, acetonitrile (30:70, v/v) at a flow rate of 1 ml/min.
% Entrapment efficiency = 𝐴𝑐𝑡𝑢𝑎𝑙 𝑎𝑚𝑜𝑢𝑛𝑡 𝑜𝑓 𝑑𝑟𝑢𝑔 𝑙𝑜𝑎𝑑𝑒𝑑 𝑖𝑛 𝑛𝑎𝑛𝑜𝑝𝑎𝑟𝑡𝑖𝑐𝑙𝑒𝑠/𝐴𝑐𝑡𝑢𝑎𝑙 𝑎𝑚𝑜𝑢𝑛𝑡 𝑜𝑓 𝑑𝑟𝑢𝑔 𝑢𝑠𝑒𝑑 𝑓𝑜𝑟 𝑃𝑍−𝑁𝑃𝑠 𝑝𝑟𝑒𝑝𝑎𝑟𝑎𝑡𝑖𝑜𝑛 * 100
The in vitro release study was carried out triplicate for both the free pazopanib solution as well as PZ-NPs (MWCO: 10,000 Da) in 20 mL of release medium PBS (pH 7.4) containing 0.1% (v/v) Tween 80 (C. Zhang, Qineng, & Zhang, 2004) at 35 ± 2°C with continuous stirring at 150 rpm. Briefly, Free pazopanib solution (1 mg/mL) was prepared by dissolving pazopanib drug in DMSO. Samples were taken 0.5 mL at 0.25, 0.5, 1, 2, 4, 6, 12, 24, 48, 72, 96, 120, 144 and 168 h. The volumes were made up with fresh release medium after each sampling. The Quantitative analysis of PZ in a sample was determined by HPLC at 260 nm to determine the cumulative percent release of drug at each time point.
Results and Discussion
Particle size and morphology
The average particle size was observed to be approximately 135 nm with a polydispersity index (PDI) of 0.131, indicating uniform particle size distribution. TEM images (Figure 1) showed spherical and non-aggregated nanoparticles, verifying the formulation process's efficacy.
Zeta Potential analysis
The zeta potential of -20.12 m V indicated good colloidal stability, essential for prolonged circulation time and enhanced biodistribution.
Encapsulation efficiency
The encapsulation efficiency of Pazopanib in PLGA nanoparticles was 33.9 ± 2.5%, achieved by optimizing the polymer-to-drug ratio. This relatively high encapsulation efficiency suggests effective loading of the hydrophobic drug within the nanoparticles.
In vitro drug release study
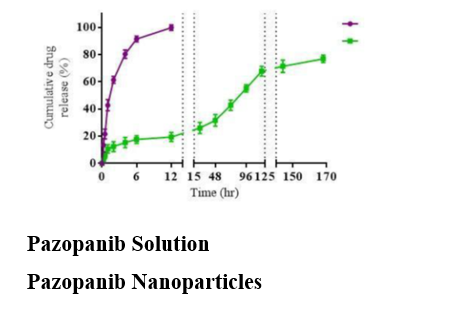
The release profile indicated sustained release of Pazopanib from the nanoparticles over 7 days (Figure 3), with an initial burst release followed by a steady release phase. This biphasic release pattern supports the hypothesis that PLGA nanoparticles can maintain prolonged drug presence at the target site.10, 11, 12
Comparative analysis with free drug solution
Free Pazopanib demonstrated rapid release, reaching nearly 100% releases within 12 hours, contrasting sharply with the nanoparticulate formulation, which released the drug gradually. 13 This sustained release can reduce dosing frequency and mitigate the systemic side effects associated with high doses.
Conclusion
The Pazopanib-loaded PLGA nanoparticles exhibited optimal particle size, good encapsulation efficiency, and sustained release properties, suggesting that this formulation could improve Pazopanib’s bioavailability while minimizing systemic toxicity. These findings contribute to the potential clinical use of PLGA nanoparticles for targeted cancer therapy.